81
|
Answers:
| molecule |
|
idealized line spectrum |
| RCH2-CH3 |
|
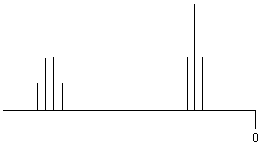 |
| RCH2-CH2R' |
|
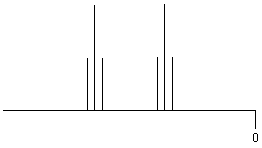 |
| RCH2-CHR2' |
|
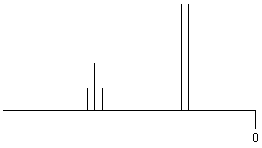 |
| RCH2-CR3' |
|
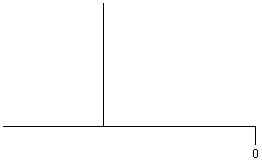 |
Any mistakes? Then check here
for some hints and explanations!
A measurement for the intensity of the coupling
between two groups A and X of equivalent protons is the coupling
constant JAX:
|
JAX |
= |
Seperation of two neighboring
lines in a multiplet, determined in Hertz |
|
As an example, the following detail of a 1H NMR
spectrum shows the doublet of the methyl protons in the molecule (CH3)3CH-O-CH(CH3)2
around d = 1.05 ppm
(Transmitter frequency n0
= 60 MHz):

What is the value for the coupling constant JCH3,CH?
Continue on page 82 for the
answer and an additional problem! |
|




