74
Make sure you really understand the following!
The splitting of the signals is caused by the through-bond spin-spin coupling.This name already indicates that the nuclear spins of different atoms (we are only considering protons at this point) interact with each other via their magnetic fields. This coupling betwen the nuclei is mediated by the electrons of the bonds that lie between the protons and their respective magnetic fields.
An example:
The two protons in dichloroacetaldehyde
| Cl2CH | - | CHO |
| X | A |
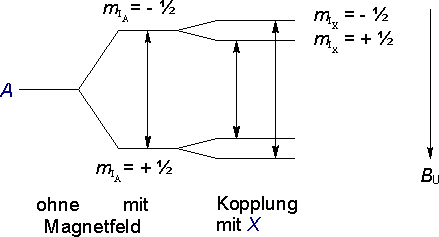
The external field B0 leads to a splitting of the energy levels of the proton A due to the alignment of the nuclear spin parallel or antiparallel to the external field. This gives rise to the possibility of transitions between the two energy levels - the resonance signals we observe in NMR spectroscopy.
 |
!!! |
Instead of a single transition, there are now two possible transitions - a doublet. One neighboring proton therefore gives rise to a doublet.
(nX = 1, therefore MA = 2).
As an interesting aside - the nearly equal intensities of the two lines in a doublet illustrates how minute the differences in population between the two states mIX = + ½ and mIX = - ½ are under normal experimental conditions.
| Now, what is the fine structure of the resonance signal of proton X? Check at page 76 for the right answer. |