72
5. Through-bond spin-spin coupling
If you want to use NMR spectroscopy in the investigation of the structure of molecules, ther are additional fine structure features of the spectra that can help you to determine the conformation of the molecule in question.At high enough resolution certain signals do not appear as simple lines, but as multiplets of closely spaced lines with characteristic patterns of intensities.
Here is an example:
The molecule we started out with CH3-CH2-CO-CH2-CH3 should have only two signals in its proton NMR spectrum:| a) | 2 -CH3 | d = 0.6 ...1.9 ppm |
| b) | 2 -CH2-CO | d = 1.9 ... 3.2 ppm. |
The actual spectrum does confirm these predictions:
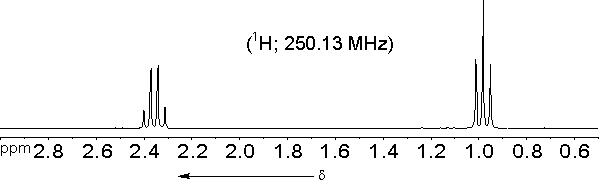
However, at closer inspection the signal of the methyl protons turns out to be composed of three lines (a triplet) with relative intensities of approximately 1 : 2 : 1, while the signal of the methylene protons is composed of four lines (a quartet) with relative intensities of approximately 1 : 3 : 3 : 1
Try to come up with a general explanation for
these experimental results by answering the following questions:
|