65
Answers:
| Isomer | Number of signals | Equivalent protons |
|---|---|---|
A)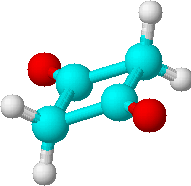 |
1 | 2 * CH2 |
B)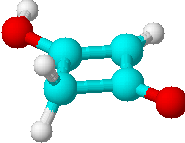 |
3 | a) >CH2 |
| b) =CH- | ||
| c) -OH | ||
C)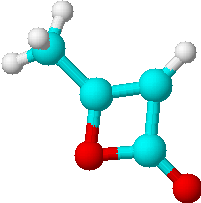 |
2 | a) -CH3 |
| b) =CH- | ||
D)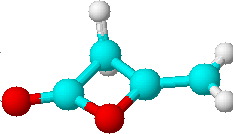 |
2 | a) >C=CH2 |
| b) >CH2 |
Did you have trouble determining the number of proton signals?
Go back to page 8 to refresh your memory!
Just by determining the number of lines expected for all possible structures of the di-ketene we could already limit our choice of solutions to the two isomers C) and D). Only they would have two lines in their proton NMR spectra. To decide which of the two structures is the correct one, we will have to look at the relative intensities of the lines.
The principles based on which we can distinguish between these two options have been discribed before (page 53):
The relative intensity of a signal is determined by the number of equivalent protons that contribute to this signal.
In our case the two signals of the di-ketene show the same intensity. Finding the answer to our question is now rather straight forward:
| Which of the two structures C)
or D) represents the true structure of the
di-ketene in question? Compare your answer with the correct solution on page 66! |