120
Answer:
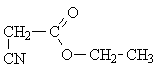
Reasoning:
As you should know by now, there are two approaches to solve this type of problem:- Starting with the experimental spectrum, you try to derive as many characeristics of the molecule that gave rise to it as necessary to come up with an unambigious structure
- Based on the composition of the molecule you make up a complete list of all the possible isomers. You then develop the theoretical 1H-NMR spectra of all the isomers and compare them to the experimental spectrum.
- Determine the corresponding multiplets, i.e.,
the signals of groups of protons that couple with each other. These
pairs (or triples etc.) of multiplets should exhibit the same coupling
constant J in the fine structure of their peaks.
In our case that would be the quartet and the triplet. - Identification the structural element that gives rise
to these coupled signals. In other words, determine the number of
protons in the groups of equivalent protons that couple to each other.
In the case of the quartet and the triplet, that would be
-CH2-CH3. - Remove this identified element from the chemical
formula. Then try to idenitify the signals of the remaining protons in
the spectrum.
After removing the cyano group -CN and the carboxyl group -COO- (Their presence has been previously determined by chemical analysis (see the problem set) as well as the identified structural element CH2-CH3 from C5H7O2N, we are left with the group -CH2- (with a chemical shift d = 3.7 ppm). - Analysis of the signal integrals to determine the
number of the structural elements determined.
In this case it is only necessary as a second check of our results so far. - Combination of the identified structural elements as
well as the elements that are based on the chemical composition and
behavior, but which do not show signals in the 1H-NMR
spectrum. This may lead to a unique structure, or - as in our case - to
two or more possible structures. Now we can use chemical shifts,
intensities and coupling costants to select the correct structure from
the two candidates
a) CH3-CH2-CO-O-CH2-CN and b) NC-CH2-CO-O-CH2-CH3
The crucial information in this case is the chemical shift of the -CH2- protons in the -CH2-CH3 group.
a) dCH2-CO = 1.9 ...3.2 ppm b) dCH2-O- = 3.4 ... 4.5 ppm.
The experimental value is 3.7 ppm, which is in good agreement with the chemical shift expected for an ether group.
Now on to the next problem!