118
Answers:
| Spectrum (A) | Spectrum (B) | |
|---|---|---|
 |
 |
Reasoning:
Based on the composition, we can derive the following isomers:| 1 |  |
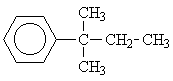
2 |  |
|||
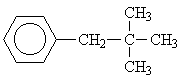
| 3 |  |
4 | 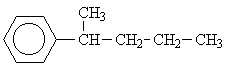 |
|||
| 5 | 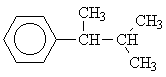 |
6 | 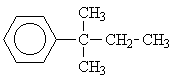 |
|||
| 7 | 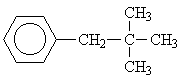 |
8 | 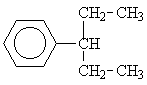 |
Spectrum (A)
The characteristic multiplicity of the signals in the spectral range d = 0.5 ... 2 ppm (A triplet and a quartet) can only be caused by a structure of the type| CH3-CH2- |
This motif occurs in the isomers 1, 3, 4, 6 and 8. But only for the isomers 6 and 8 would we expect three signals from the aliphatic side chains.
(E.g., in the case of the isomer 2 we would expect signals from five non-equivalent groups of protons in this spectral range)
To distinguish between the two remaining options, we will have to consider the single signal at d = 1.25 ppm. Since this signal is a singulet (Multiplicity M = 1), only isomer 6 could give rise to it.
In this molecule the methyls CH3 of the group
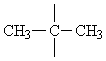
are equivalent and ouple with no other group, thus being represented by only a single signal in the spectrum.
Spectrum (B)
Matching this spectrum to isomer 7 is rather straight forward based on the number of aliphatic signals (2 groups of equivalent protons) and the lack of spin-spin coupling between the signals.Now continue to the next problem!