25
Great! Right answer!
Indeed, the three protons of the methyl group and the single proton of the carbonyl group (-CH3 und -CHO) give rise to two signals. (If you want to, you can check out the reasons for this on page20)The same reasoning holds for the two protons of the methylene group (-CH2-), since they also rotate freely, and since the two protons have mirror symmetry. They also give only one shared signal.

C1 and C3 rotate for all concerns and purposes freely around the single bonds C1-C2 and C2-C3, respectively. Therefore both methylene protons experience the same average amount of shielding over time from the CH3- and the CHO- groups.
To summarize: In systems with unrestricted movement all the protons of aComplications with regard to this rule only arise when the rotation around the single bond produces conformations that are related through a symmetry operations (e.g., when the CH2- group is next to an asymmetrical carbon atom).produce only a single, shared resonance signal.
- methyl group (-CH3)
- methylene group (-CH2-)
You can find further explanations of this on page 132!
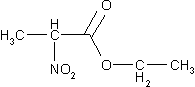
Now try to determine the expected number of
resonnance signals for the following molecules:
Compare your answers with the solutions! |